Zoltán Kiss- Area Sales Manager - East Europe - Endrich GmbH.
Primary lithium metal batteries from leading manufacturer EVE Battery
7 September 2015

Summary :
To find energizing solution for potable electronics devices is a challenge, it is definitively a serious consideration to select the right primary battery no matter if we design the device or we are the user of device. The disposable batteries available on the market today are manufactured in various form factors, and usually their chemical systems are completely different. The old carbon-zinc batteries have been replaced by well know alkaline batteries, and today the focus of engineers turn to the lithium batteries, that are lightweight, more durable and chargeable than everyday batteries. To support selection of lithium disposable batteries, we try to overview the technical possibilities, the technical features and suggested applications of the different types through the product range of the World leading lithium metal primary battery manufacturer EVE Energy.
Lithium technology– disposable and rechargeable batteries
Battery is an energy storage system, that converts chemical energy to electronic energy due to its capability to generate electric charge by chemical reaction, The chemical reaction creates free electrons, that can flow as electric current, that we can use to energize circuit placed between anode and cathode. If the chemical reaction is not reversible after the discharge of the battery, one of the material of the reaction runs out, we talk about primary or disposable device, that should be replaced. If the charge can be recovered by using external electric current, we talk about rechargeable or secondary battery.
During the technology development the feasibility to use lithium as the lightest, lowest density metal, with highest electrochemical potential and best energy storage/weight ratio came into view. Technical literature uses ‘lithium or lithium metal batteries’ phrase for the disposable types with lithium anode. These are not to be mixed up with the lithium-ion rechargeable accumulators , that have no lithium anode, but graphite, and their cathode is a composite of lithium and some transition metal-oxide like nickel, cobalt, manganese or iron oxide. Usually the electrolyte is a lithium salt dissolved in a kind of organic carbon solvent. When discharging, the rapid reagent lithium gives up one of its electrons, and transforms into Li+ ion, the free electron flow results the electric current. When recharging, the Li+ ions flow back and intercalate into the anode’s porous graphite material, forced by the external voltage caused electromotive force, and the system will be ready again to generate electrical energy. Next to the conventional Lithium-Ion accumulators, at the end of the nineties new type of rechargeable batteries came into focus, the so called lithium-poly families, that use solid polymer composite electrolyte instead of liquid, and instead of the hard metal case of Li-Ion types, these devices have flexible cover shell, and manufactured in diverse tiny sizes. Although their capacity is usually smaller, they can be better used in portable electronics. EVE uses both Li-Ion and Li-Poly technology in its own rechargeable battery production, but real leading role EVE Energy has on the market of disposable primary batteries.
General properties of lithium-metal primary batteries
Temperature/Humidity
The biggest enemy of batteries is heat, the self discharge of primary batteries stored on high temperatures may grow as high as 35%. Ideal storage conditions are characterized by humidity between 40-90% and temperature range of +10°C to +25°.
Nominal capacity
By definition nominal capacity is the multiplication of discharge current (A) and the time to discharge (h).
C= I (A) * t (h)
Capacity is the charge value that is available during discharging from fully charged status to the moment the breakdown voltage is reached on given discharge condition (discharge current & C-rate )
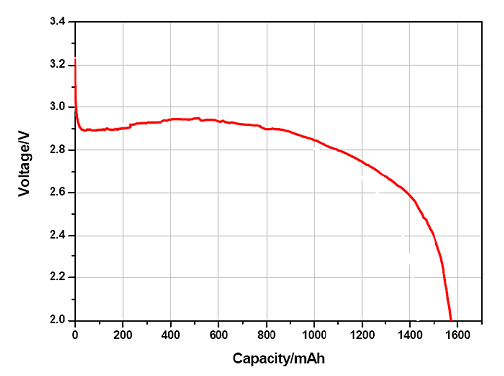
Battery voltage
To describe voltage of battery on a satisfactory way, we need to define several different voltages, that all characterize battery behavior. Nominal voltage is the primary reference voltage, however in practice we have to distinguish between voltage without load, the open circuit voltage (OCV) and during discharging the close circuit voltage (CCV). The voltage value, where the battery is considered fully discharged, is called the breakdown or cut-off voltage.


Passivation
Passivation is a phenomenon of lithium primary cells related to the interaction of the lithium metal anode and the electrolyte. A thin so called passivation layer forms on the surface of the anode at the moment the electrolyte is injected into the cell during production. This layer is important because it protects the anode from reaction while the cell is not affected by load, resulting in a long shelf-life. Under load, when battery starts to discharge, the current flowing through the cell will start to rebuilt this layer. Under normal conditions, the thin passivation layer does not affect or degrade the performance of the battery cell. When the layer grows too thick due to long storage, discharge performance may be affected. The development of the passivation layer is influenced by the conditions of the storage, long unloaded periods of months or years and keeping the cells above room temperature (23-25 oC) will cause the passivation layer to grow thicker. A passivated cell may show voltage delay when suddenly applied under load, the voltage response is delayed. As the passivation layer thickens, the voltage delay becomes more severe. On continued discharge though, the voltage of a passivated cell returns to the level of an unpassivated cell. At low discharge currents the wake up time of the cell is acceptable, since the voltage response arrives in time, however if cell has to serve high pulse current, the voltage may decrease under cut-off voltage.
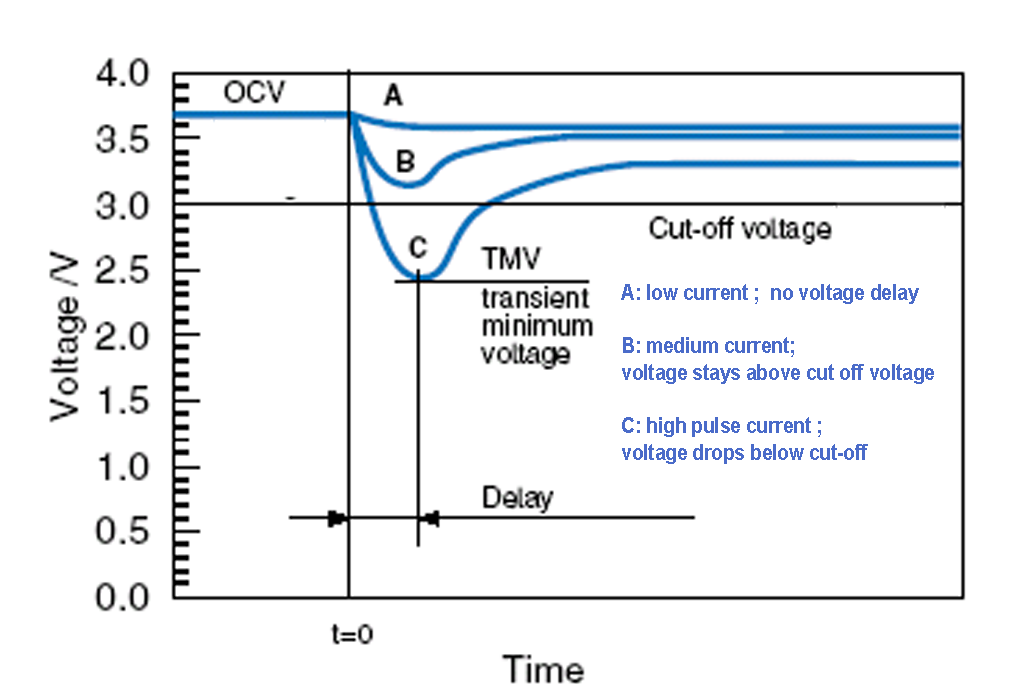
Providing adequate storage conditions to reduce the chance of passivation is the best way to eliminate voltage delay problems. There are several effective other methods for dealing with excessive passivation when storage conditions cannot be controlled. The layer can be kept from growing too thick by maintaining a light load on the cell during storage. Alternatively, a high load, placed on the cell at regular intervals during storage can be a solution. Applying intelligent programmed start using load just before the anticipated start-up of the cell can also be used to burst the passivation layer and restore normal performance. All of these methods will have an impact on the capacity of the cell. In particular, a low rate discharge tends to increase the normal self-discharge reaction of the cell and reduce the available capacity. >
In spite of the problem of the voltage delay, it would be a mistake to consider passivation as a harmful property, as this is the phenomenon that provides lithium primary batteries with extreme long shelf life time. We will detail later a special type of lithium metal batteries, the LiMnO2 CR batteries, that do not suffer from passivation even in case of long storage of years or exposure of excess heat. Other lithium primary systems have to be ideally kept under low and continuous load.
Internal structure
Internal structure of primary lithium batteries is also to be seriously considered, as their affect on battery operation is significant. The cylindrical LiSOCl2 (ER) batteries have either spiral or so called bobbin structure. Spiral ones are constructed of a high surface metallic foil as an electrode wounded around a core in order to reach high impulse currents, while bobbin type is made as a wire bobbin to store more energy.
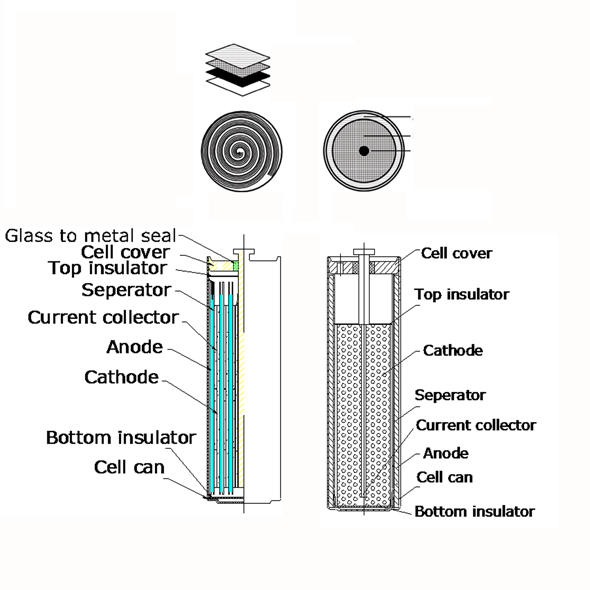
In spiral cells the more space the spiral electrodes take, the less space remains for electrolyte, therefore energy storage capacity is lower, but due to the huge electrode surface their pulse capabilities are advanced. In bobbin cells, the energy storage capacity is 30% higher than spiral versions due to more electrolyte in the can, although the maximum pulse current is low. If design needs high momentary current provided within a short time, spiral cells have more advantages, while in cases high capacity is a must, bobbin types are better to be used. As a matter of fact spiral cells could be dangerous when physical damage occurs due to their high impulse energy delivery capability. Although EVE constructs its spiral ER batteries with well designed safety vents in order to avoid accidents, it is safer to use bobbin type cell and an additional SPC device in high energy packs. SPC is a device that is connected parallel with ER battery and capable to pump charge with high pulse current to the circuit, while energy is stored in the safer bobbin cell.
Spiral cells have advantage in less voltage delay, as suffer less of passivation than bobbin versions. Above introduced special pack with bobbin cell and additional SPC will however solve this issue, as the SPC is able to pump charge immediately to the system while bobbin type of ER battery is about to wake up in the background.
Part numbering rules
Battery manufacturers follow concerning standards, so it’s quite easy to compare products of different manufacturers. In order to complete the image, we introduce the naming conventions in below table. Primary cells are differentiated by their chemical structure at first, as the first letter of the product code represents.
| (-) | Electrolyte | (+) | |
|---|---|---|---|
| - | Zn | Ammonium chloride; Zinc chloride | MnO2 Manganese –dioxide |
| A | Zn | Ammonium chloride; Zinc chloride | O2 Oxygen |
| B | Li | Organic electrolyte | CFx carbon–monofluoride |
| C | Li | Organic electrolyte | MnO2 Manganese-dioxide |
| E | Li | Non-aqueous inorganic electrolyte | SOCl2 Thionyl chloride |
| F | Li | Organic electrolyte | FeS2 iron-disulfide |
The second letter is for the shape, and the resit represent the size
| R | Round shape or button / cylindrical cell |
| F | Flat cell |
| S | Square /Rectangular/ Prismatic cell |
| Type | Diameter | Height | Width | Thickness |
|---|---|---|---|---|
| CR2032R | 20 | 3.2 | - | - |
| CF502445 | - | 5,0 | 24,0 | 45,0 |
EVE Battery’s primary families that we would like to introduce in details are the followings:
- Li-SoCl2 – Lithium thionyl chloride ERxx/EFxx
- Li-MnO2 – Lithium manganese dioxide CRxx/CFxx
- Li-FeS2 – lithium iron disulfide AA/AAA
Li-SoCl2 – Lithium thionyl chloride ”ER” batteries
As the chemical structure regards, lithium thionly chloride cells have a metallic lithium carbon - the lightest of all the metals – anode and a liquid cathode comprising a porous current collector filled with thionyl chloride (SoCl2). The chemical reaction occuring during discharge is as follows :
4Li + 2SOCl2 → SO2 + S + 4LiCl
They deliver a nominal voltage of 3.6V, their open circuit voltage is 3,66V and during load with their 3.4-3,6V closed circuit voltage they are one of market’s highest voltage primer cells. Available in various cylindrical shape, in 1/2AA to D format, with spiral electrodes (e.g. l ER14250M) for power applications and bobbin construction (e.g. ER14250) for prolonged discharge. Lithium thionly chloride batteries are the primary battery currently with the highest voltage and energy density (1280 Wh/dm3), longest storage (10-20 years) , and the least self-discharge rate of 1%@20°C. The battery is capable of operation in a wide temperature range normally from -60°C~+85°C. One special has an extended temperature range up to 150°C. Those batteries are ideal for such long-term applications as power for electric devices and electric power, water, heat and gas meters, and especially as backup power source for memory ICS.
| Battery type | OCV(V) | CCV(V) | Cut off (V) | |
|---|---|---|---|---|
| Lithium battery | Li/SOCl2 | 3.67 | 3.6~3.3 | 2 |
| Li/SO2 | 3.1 | 2.9~3.1 | 2 | |
| Li/MnO2 | 3.5 | 2.8~2.7 | 2 | |
| Li/CuF2 | 3.1 | 2.7~2.6 | 2 | |
| Li/CuO | 1.5 | 1.6~1.4 | 0.9 | |
| Other primary battery | Zn/HgO | 1.35 | 1.6~1.2 | 0.9 |
| Zn/MnO2 Alkalescence | 1.5 | 1.25~1.15 | 0.9 | |
| Zn/MnO2 Neutrality | 1.5~1.75 | 1.25~1.15 | 0.9 | |
UN and UL approvals guarantee safety in delivery and in application.
The capacity of the battery determines the time to discharge on a certain discharge current level. During discharge the working voltage of the cell starts decreasing, and at the moment reaching the cut-off voltage, the cell is considered empty.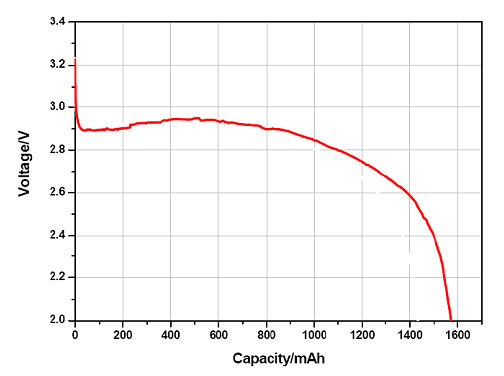
When design-in ER batteries, the affect of ambient temperature to cell behavior should be kept in consideration, as this has huge influence on discharge characteristics. Below figures show the different temperature-voltage characteristics of the two basic structural types of ER batteries (bobbin ER17505 and spiral ER17505M) with different discharge currents.
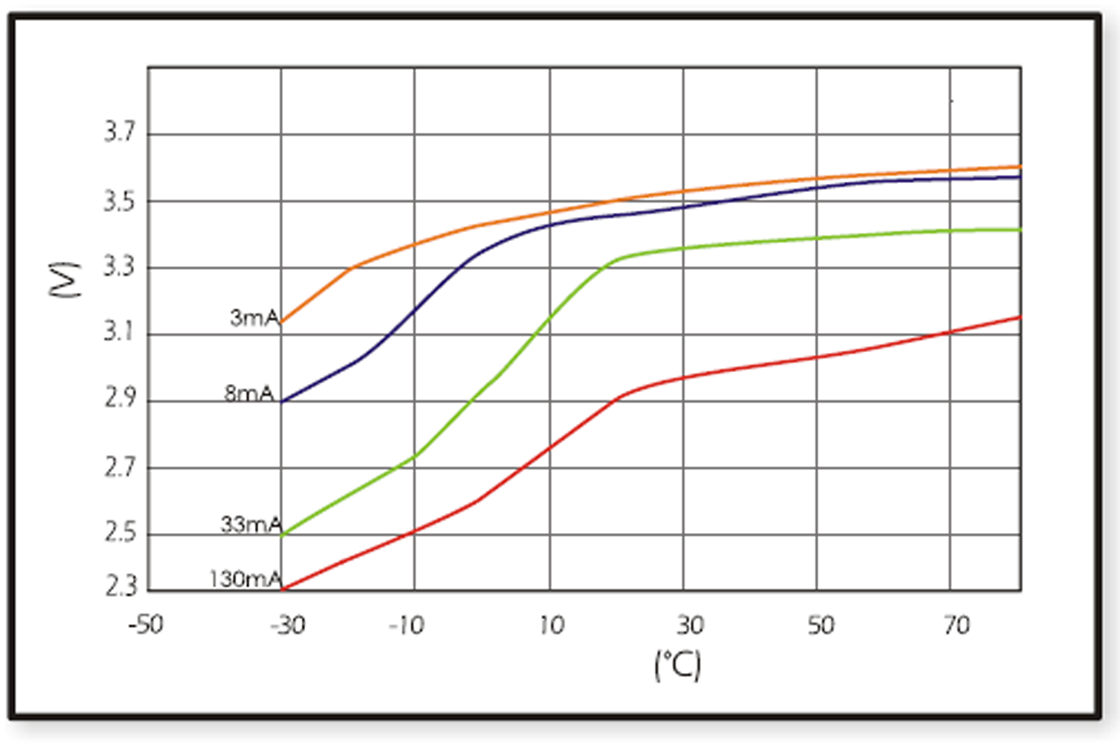
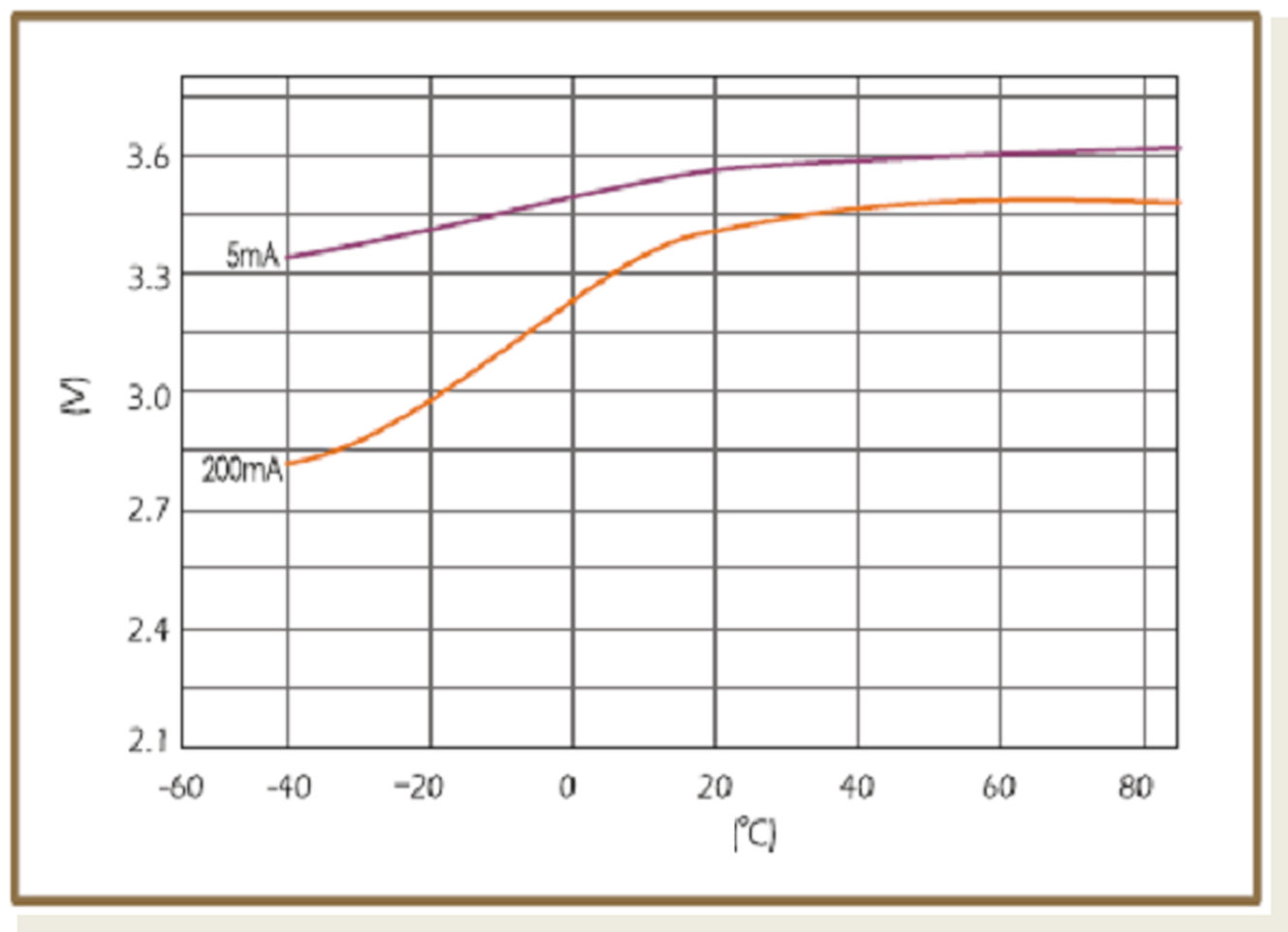
Spiral cells show less sensitivity to changes of ambient temperature than bobbin type of batteries. They suffer also less from passivation, but their capacity is lower.
For high temperature application such as energizing sensors in mineral or oil drilling heads, EVE also produces ER batteries with extreme shock proof and extended temperature range stainless steel encapsulation (ER14250MR-150).

Typical application of ER batteries:
- Smart utility metering (Electricity, gas, heat , water)
- Alarms and security devices
- GPS, RFID applications, vehicle tracking
- Automotive telematics systems
- Professional electronics
- Oil exploration
- Military radio communication
- Outdoor sensors
- Emergency location transmitter beacons (ELT,EPIRB)
| Spiral (ErxxM) | Bobbin (Erxx) | |
|---|---|---|
| Capacity |
- |
+ |
| Pulse current |
+ |
- |
| Passivation |
+ |
- |
| Security |
- |
+ |
Just to be mentioned as a curiosity, EVE produced already in 2014 daily 400.000 pieces of ER batteries, mainly cylindrical types.
Li-MnO2 – Lithium manganese dioxide ”CR” batteries
As the chemical structure regards, lithium manganese dioxide cells have a metallic lithium - the lightest of all the metals – anode and a solid manganese dioxide cathode, immersed in a non.coorosive, non toxic organic liquid electrolyte. The chemical reaction occuring during discharge is as follows :
Li + MnIVO2 –> MnIIIO2 (Li+)
When lithium transfers into Li+ ion electrons are released, and these electrons– in closed circuit – flow freely as electric current providing transformation of chemical into electrical energy. The cell voltage of CR batteries is 3V (OCV= 3.1..3.4V CCV=3,0V), operation temperature range is -40°C -+85°C when cylindrical , and -20°C-+70°C when coin structure. Automotive industry favors he usage of extended temperature range -40-+125°C versions, primarily for TPMS (tire pressure management systems). In spite of the fact that CR batteries’ energy density is beyond ER batteries’ one, they feature many advantages , as no need for protective circuits, and no need to take care of passivation typical to the liquid cathode systems. As there are no cadmium, mercury and lead contents it is more economy friendly. Self discharge is less than 1%@20°C, so the storage life is extremely long up to 10 years when providing ideal storage conditions. Best to use these kind of batteries, when application needs thin, small size, light energy source for lower loads. Next to he cylindrical and button types, there is also a 9V battery at EVE’s program, that is constructed by using three serial connected 3V cells packed into a rectangular case.
EVE produces in average daily 720,000 pcs of CR batteries, most of them are the button types.
Application of CR batteries :
- Computer main boards; CMOS and RTC (real time clock)
- Car ignition keys, remote controls
- Sensors of dangerous gases
- Medical electronics (handheld devices like blood glucose meters etc)
- Smoke detectors
- Digital cameras
- Intelligent utility metering
- RFID
- ETC (electric toll collect), TPMS
Lithium iron disulfide (Li-FeS2) disposable batteries
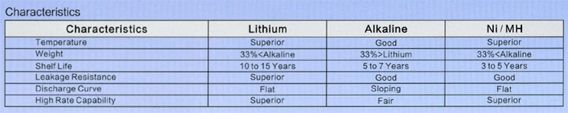
Cylindrical lithium ferrite disulfide (Li-FeS2) have lithium anode and iron disulfide cathode , the electrolyte is a lithium salt blended in an organic solvent. Cell voltage is 1.5V that makes the battery compatible with all kind of AA and AAA disposable batteries. Using them instead of conventional battery types has many advantages such as the working ability at extreme low temperature, excellent performance even after 15 years of storage at ambient conditions and longer service time.


Features:
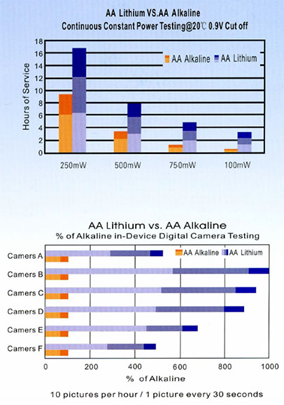
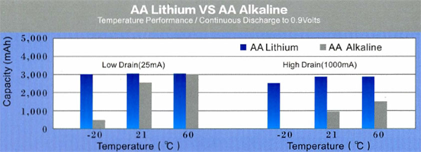
On the figures we show a comparison between and AA alkaline and a LiFeS2 battery capacity with different constant discharge current at 20°C till reaching 0.9V CutOff voltage. At high discharge current lithium performs much better, alkaline’s service time is far beyond lithium’s one. The difference is much smaller at lower discharge current, it is just remarkable at low temperature.
On extreme low temperature lithium’ advantage is remarkable no matter what discharge current is being used. On high discharge currents no matter of temperatures, lithium service time is always remarkable higher. The only conditions where the two types’ service time is comparable are the normal temperature range and small discharge current.
Application area:
| Share on Facebook | Share on LinkedIn |
References
This article has been published on the following locations:
| # | Media | Link |
|---|---|---|
| 1 | Elektronet 2015/5 | Elektronet : elektronikai informatikai szakfolyóirat, 2015. (24. évf.) 5. sz. 20-23. old. |
| 2 | Elektronet online | Eldobható lítiumelemek a világ vezető gyártójától |
| 3 | Hungarian version | Eldobható lítium elemek a világ vezető gyártójától - EVE battery |
| 4 | TechStory M2M | Eldobható lítiumelemek |
| 5 | Jövő Gyára 2015/4 | 2015. 4.sz. 43-44.o. |
| 6 | Jövő Gyára online | Lítium vas-diszulfid eldobható elemek ez EVE-től |
| 7 | New Technology online | Eldobható lítium elemek |
| 8 | New Technology 2019/5 | 2019. 5.sz. 18-20.o. |


